The different physical properties -- color taste and so on -- of materials came about because atoms in them had different shapes andor arrangements and orientations with respect to each other. The formula for water is H2O and for hydrogen peroxide is H2O2.

3 Ways To Study The Chemical And Physical Properties Of Atoms In The Periodic Table
As such there is large number of atoms per unit volume ie.
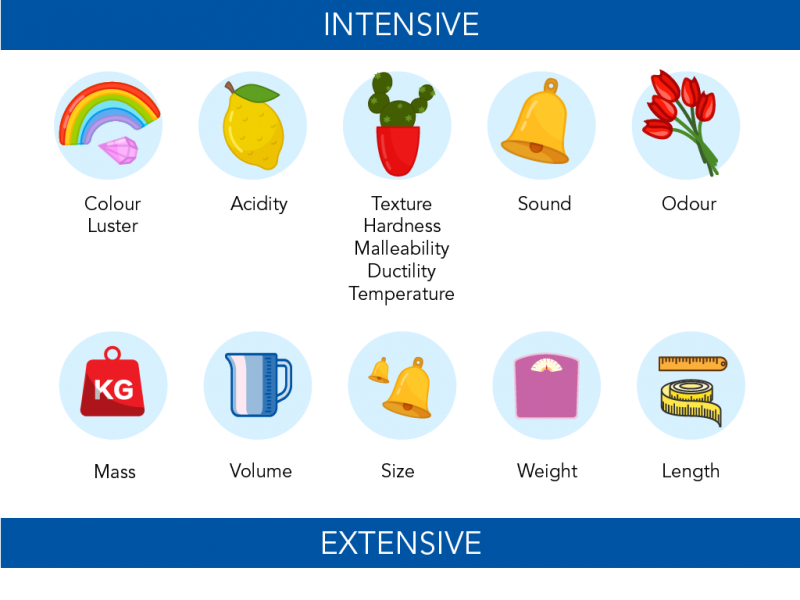
. Isotopes of an element have different physical properties because they have different mass numbers. The physical properties of any isotope are largely determined by its mass. The electromagnetic behavior of the atom is govern by a few principles but for our sake lets just look at coulumbs law which states that the attractive force between two charges is proportional to the number of charges and inversely proportional to the distance square.
It occurs when atoms share or transfer valence electrons. Isobars are elements that have different chemical properties but the same physical properties. How did Leucippus and Democritus use atoms to explain different physical properties.
Atoms all have about the same size but they have different weights. Atoms are indivisible particles that cant be destroyed or created through chemical reactions. Chemical properties of different isotopes are almost similar.
We can use this information to predict what gold will do under different conditions and to make decisions about whether or not it is good material to use when we are. The Behavior of Atoms. When it comes to physical properties of isotopes including mass melting or boiling point density and freezing point they are all different.
For example a gold coin is simply a very large number of gold atoms molded into the shape of a coin with small amounts of other contaminating elements. 2 Malleable and ductile. Do all oxygen atoms have the same properties.
H202 is an ionic bond where one or more electrons are transferred from one atom to another. Phases of Matter and the Properties of Gases. Avogadros suggestion that a volume of any gas under equal temperature and pressure contains the same number of particles led to an.
How did they use atoms to explain different physical properties. The differing properties of carbon and diamond arise from their. These physical properties can be easily explained by looking at the structures of metal.
Plutonium is one of the. The differences in the arrangement of atoms affect the properties of the material. The way the carbon atoms are arranged in space however is different for the three materials making them allotropes of carbon.
Elements form compounds when they combine chemically. Law of Multiple Proportions. It can not explain the rigidity of the metal atom.
Covalent bonds are also not possible for metal. Chemical bonds are the electrical forces of attraction that hold atoms or ions together to form molecules. When two elements get together they form a compound.
Physical Properties of Metals 1 Usually have high densities. Atoms in a metal are packed tightly in layers and are held together by strong metallic bonds. Different materials can be made from atoms of the same element.
H20 is a covalent bond where moving electrons travel about in the nuclei of both atoms. Transparency electrical properties such as conductivity physical properties such as density and boiling point and chemical properties such as reactivities and reaction rates. A chemical bond is a force of attraction between atoms or ions.
When the atoms in a metal are identical they can not show ionic properties because the ionic compounds are formed between two different atoms. Atoms combine in a ratio of small whole numbers to form compounds. The isobars have the same atomic mass but a different atomic number because the discrepancy in the number of nucleons is compensated by an increased number.
They used color taste etc- of materials came about because atoms in them had different shapes andor arrangements and orientations with respect to each other. But they have different numbers of neutrons and so the atomic masses are different and so are some physical properties. Water and hydrogen peroxide both consist of hydrogen and oxygen atoms.
An atom is the smallest unit of matter that retains all of the chemical properties of an element. In covalent compounds much weak Van der Waals force acting between the two bonding chemical atoms. Their atoms join together to form molecules crystals or other structures.
If this atom is part of a. Naphthalene C_10H_8 bp 218 C mp. By documenting how particles behaved in different states of matter 19th century scientists gained a deeper understanding of the atom.
So we can define isobars as elements having different atomic numbers but the same mass number. Physical Properties and Intermolecular Forces The physical state and properties of a particular compound depend in large part on the type of chemical bonding it displays. In a molecule of water two hydrogen atoms are chemically bonded to one oxygen atom whereas in one molecule of hydrogen peroxide.
Rationalize the difference in physical properties In terms of intermolecular forces for the following organic compounds benzene. Gold atoms cannot be broken down into anything smaller while still retaining the properties. The atoms are held together by chemical bonds.
Explain why they have different chemical and physical properties. Different number of particles neutrons protons and electrons is the reason why they behave so different. Well known elements are hydrogen oxygen iron or lead.
The properties of the material depend upon the bonding between the atoms and structure of the substance. Different types of chemical bonds and their varying intensity are directly responsible for some of the physical properties of minerals such as hardness melting and boiling points solubility and conductivity. All atoms of an element have identical chemical properties and mass whereas atoms of different elements have different chemical properties and masses.
There are over a hundred different types of atoms that we call elements. C_6H_6 bp 80 C mp 6 C. Confusing but basically the ratio of each element decides the structure and thereby properties.
The Law of Multiple Proportions states that when 2 elements form a series of compounds the ratios of the masses of the second element that combine with 1 gram of the first element can always be reduced to small whole numbers. For example water has two atoms of hydrogen and one atom of oxygen. They form the world we live in.
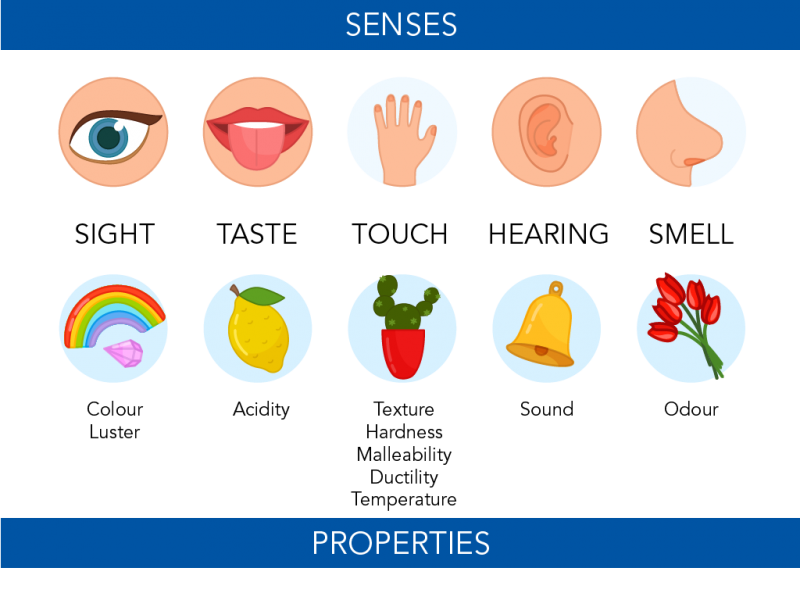
Describing And Classifying Matter Let S Talk Science

Describing And Classifying Matter Let S Talk Science

3 Ways To Study The Chemical And Physical Properties Of Atoms In The Periodic Table
0 Comments